CBSE Class 10 Chemistry - Carbon and Its Compounds - Very Short Answer Based Questions on Allotropes (Part -2)
Q1: What is the atomic number of a carbon atom?
Answer: 6
Q2: How many valence electrons are present in a carbon atom?
Answer: 4 (electronic configuration is K:2 L:4)
Q3: What are allotropes?
Answer: The property due to which one element exists in more than one form, which differs in physical properties but has similar chemical properties. These forms of an element are called allotropes.
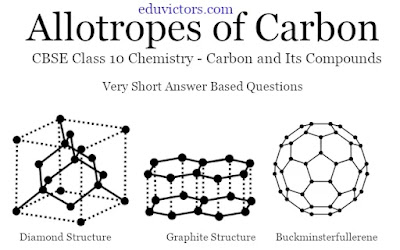
Q4: Name three crystalline allotropes of carbon.
Answer: Diamond, Graphite and Fullerene
Q5: What is the arrangement of carbon atoms in diamond?
Answer: Rigid three-dimensional network structure.
Q6: Is diamond a good conductor of electricity?
Answer: No. It is a poor conductor. Because all free electrons are firmly held in C-C bonds.
Q7: Is diamond a hard or a soft substance?
Answer: Hard (In fact one of the hardest substances in nature.)
Q8: Name the allotrope of carbon used in cutting gemstones, marbles, glass etc.
Answer: Diamond
Q9: Is diamond a good conductor of heat?
Answer: Yes. In fact, its thermal conductivity is 5 times that of copper.
Q10: It is soft, greyish black in colour and is used in making pencil leads. Name the allotrope of carbon we are talking about?
Answer: Graphite
Q11: What is the geometrical structure of graphite?
Answer: Two-dimensional sheet-like.
Q12: Is graphite soft and greasy?
Answer: Yes, that's why it is used as a lubricant.
Q13: Which allotrope is called plumbago or black lead?
Answer: Graphite
Q14: Is graphite a good conductor of heat and electricity?
Answer: Yes. That's why it is used in making electrodes in electrolytic cells.
Q15: Name the allotrope that has a shape of a soccer ball (football)?
Answer: Fullerene
Q16: How many atoms exist in the smallest fullerene molecule?
Answer: 60 carbon atoms.
Q17(HOTS): Do other elements exhibit allotropes?
Answer: Yes. For examples allotropes of phosphorus are:
i. White phosphorus
ii. Red phosphorus
iii. Black phosphorus
Q18 (NTSE/ JEE / NEET): Name the eight allotropes of carbon.
Answer: Eight allotropes of carbon:
(a) diamond,
(b) graphite,
(c) lonsdaleite,
(d) C60 buckminsterfullerene,
(e) C540 fullerite
(f) C70 fullerene,
(g) amorphous carbon,
(h) zig-zag single-walled carbon nanotube.
👉See Also:
Ch 4 - Carbon & Its Compounds (Short Q & A)
Ch 4 - Carbon & Its Compounds (Q & A)
Ch 4 - Carbon & Its Compounds (MCQs)
Ch 4 - Carbon & Its Compounds (Eduvictors' Quiz)
Ch 4 - Soaps and Detergents
Ch 4 - Carbon & Its Compounds (Important Points To Memorise)
Ch 4 - Carbon and Its Compounds (PDF Notes)
Ch 4 - Carbon and Its Compounds Part-1(VSQA)
No comments:
Post a Comment
We love to hear your thoughts about this post!
Note: only a member of this blog may post a comment.